Abstract
Background: Complete remission (CR) rate and long-term overall survival (OS) is very poor in patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (R/R BCP-ALL). Previous clinical trials revealed a good remission rate and a safe bridging role to allogeneic-HCT compared to various standard treatments, and several real-world data have been reported.
Aim: Here, we analyzed outcome of blinatumomab versus our conventional intensive chemotherapy by propensity score-matched analysis.
Methods: From 2009 to 2020, 197 consecutive R/R BCP-ALL were treated with mitoxantrone-etoposide-cytarabine (MEC, n=113) or blinatumomab (n=84), and allogeneic-HCT was planned in patients with CR. For propensity score-matching, the MEC and blinatumomab cohorts were matched in a 1:1 ratio using 5 criteria of age < 35 years old, short CR duration < 12 months, adverse-risk karyotype including Ph-chromosome at relapse, previous allogeneic-HCT, and first-line salvage or not. We compared CR rate, regiment related mortality, and OS with cumulative incidence of relapse (CIR) and non-relapse mortality (NRM).
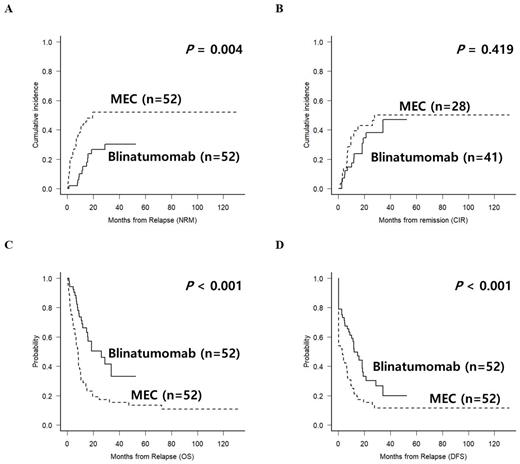
Results: Matched cohort consisted of 52 patients for each salvage group and matched criteria were the same exactly. Median age was 42 years and 34 (65.4%) patients were > 35 years old. There were 22 (42.3%) primary refractoriness, 9 (17.3%) post-chemotherapy relapse and 21 (40.4%) post-HCT relapse. Among 30 relapsed patients, 19 (63.3%) were early relapse with CR duration less than 12 months. There were 25 (48.1%) with poor-risk karyotype at relapse of which 10 were Philadelphia-chromosome. Extramedullary relapse was observed in 14 (26.9%) and 8 (15.4%) were advanced-line salvage. CR rate was significantly higher in blinatumomab (78.8% vs. 53.8%, p=0.012) and more patients proceeded to allo-HCT (80.8% vs. 46.2%, p<0.001). Regimen-related mortality was significantly higher in MEC (40.4% vs. 1.9%, p<0.001). After median follow-up of 32.5 months (range 6.2 to 131.2), 3-year OS of blinatumomab and MEC was 33.2% and 15.4% (p<0.001), and 3-year NRM was 30.3% and 51.9% (p=0.004), respectively. After CR achievement, CIR was not significantly different (46.9% vs. 60.0%). In multivariate analysis, CR duration < 12 months was related with poor OS with high CIR. MEC regimen showed poor OS with high NRM. High NRM was also related with advanced-line salvage.
Conclusion: Our matched cohort analysis clearly showed that blinatumomab is superior to MEC salvage regimen. However, we are observing relapse in up to 50% after blinatumomab and following allo-HCT still shows high NRM. Further combinatorial using novel therapeutics are needed for R/R BCP-ALL.
Kim: AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AIMS Biosciense: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; AML-Hub: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BL & H: Research Funding; BMS & Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Boryung Pharm Co.: Consultancy; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Handok: Consultancy, Honoraria; LG Chem: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria; Pintherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Honoraria, Speakers Bureau; SL VaxiGen: Consultancy, Honoraria; VigenCell: Consultancy, Honoraria. Lee: Alexion, AstraZeneca Rare Disease: Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal